Article by: Harry T. Jones
Edited by: J. D. Dixon and Adam Manning
The preservation of colour in the fossil record was long thought impossible, but discoveries made in 2008 have since led to multiple investigations into how colour can survive fossilisation. Melanosomes (pigment-bearing organelles) found in exquisitely preserved fossils can be compared with those found in living animals to indicate the colours of extinct organisms. A similar comparison can be made with structural colours, which generate colour by nanostructures scattering light, typically found in fossil and living insects. Other indicators of colour include preserved tetrapyrrole molecules in eggshells, reptilian pigment cells, genes, and fur. Colours in fossils can suggest a lot about organisms, their behavioural patterns, and how they interacted with each other and their environments.
Melanosome Pigmentation
Melanosomes are membrane-bound organelles, which occur in soft-tissue structures, such as feathers and skin, and contain the pigment melanin. Melanins are strong polymers that tightly cross-link with proteins (including keratin found in feathers), strengthen tissues, and are believed to aid fossil feather preservation. Melanin bestows black-to-reddish-brown hues to living organisms. Studies suggest that melanosome shape indicates the type of melanin they contain and what colour that melanin induces, as their morphology varies depending on the chemical variant of melanin they enclose. Phaeomelanosomes (Figure A) are spherical melanosomes and contain reddish-brown inducing phaeomelanin. Eumelanosomes (Figure B) are rod-shaped, and contain eumelanin, which produces dark colours, such as black and brown.

The discovery of melanosomes in fossils was first reported in 2008, when scientists suggested that microscopic tube-like structures, previously labelled as fossilised bacteria, could be melanosomes. Scanning electron microscopy was used to investigate dark bands of a hundred-million-year-old fossil feather from Archaeopteryx lithographica. It was hypothesised that if the tubes were only found in the dark stripes, they may be melanosomes, but if they were bacteria they should be over the length of the feather. The “oblong bodies” were only found in the dark sections, suggesting the melanosome hypothesis was correct. Despite the evidence for these structures being melanosomes, some scientists maintain that published studies have failed to disprove the theory that they are fossil bacteria. One of their arguments is that melanosomes and many bacteria share a similar size and morphology, so cannot be differentiated by morphology alone, and bacteria are found everywhere and fossilise habitually. However, evidence for melanosomes seems to outweigh that for bacteria. For example, the arrangement of nanostructures along a feather’s barb long axis cannot be formed by bacteria as bacteria do not precisely align themselves according to feather morphology. Others argue such structures are the degraded remains of structural proteins collagen or keratin, but investigations into Sinosauropteryx tail feathers refuted claims that these structures were damaged collagen fibres, indicating melanosome presence in protofeathers.
Factors Affecting Colour
Some scepticism exists regarding the accuracy of modern depictions of extinct organisms’ colours. Melanosomes are not the only factor in determining feather colour as diet can influence bright feather colours, such as flamingo pink, meaning that even the most accurate melanosome identifications may not indicate the organism’s true feather colours. Equally, the entrapment of fossils in rock over time subjects them to the effects of diagenesis and chemical alteration. The red-brown and violet hues of feather barbs may only be a result of the diagenetic incorporation of metals, particularly iron, into melanosome sacs, so colours found today may not represent what living organisms looked like millions of years ago.
Criticisms and Development
There are also criticisms surrounding early investigations into fossilised pigments. Only one Archaeopteryx feather was studied before it was concluded that it was probably black, which has been likened to trying to determine a modern peacock’s colour from a few dozen pigments. To improve depictions, recent analyses have involved X-ray fluorescence, which causes elements to emit a specific burst of light. X-ray analysis of Confuciusornis sanctus fossil feathers has shown that copper is organically strongly bound to eumelanin, as it is in modern feathers. At high concentrations (Fig. 2B), copper becomes toxic to most bacteria, perhaps reducing degradation by microorganisms before fossilisation, suggesting that copper could be a biomarker for eumelanin.

Pigment Degradation and Experiments
Another problem with using melanosomes is that pigments chemically degrade after an animal’s death, leading to rapid changes in colouration. As melanosome morphology can change during diagenesis, making shape alone an unreliable indicator of colour, researchers have recreated the conditions in which fossils were formed by subjecting modern feathers to high pressures and temperatures to observe melanin’s chemical alteration. One study indicated that melanosomes shrink by up to 10% during burial diagenesis and maturation, and another concluded that thermal maturation has a greater impact on fossil eumelanin’s chemical preservation than age, and that eumelanin alteration is largely independent of diagenetic mineralisation.
Structural Colour
Structural colours are another source of colour in organisms, and are produced when light is scattered by nanostructures, so both an organism’s pigments and structural components are needed to generate an accurate picture of a fossil’s original colour. In insects, multilayer reflectors are the most common form of biophotonic nanostructure. Reflectance spectrophotometry was used to conclude that structures of studied fossil cuticles had survived fossilisation with little/no alteration, so the fossilised multilayer reflector could prove reliable in determining whether fossil beetles’ colours are original. Fossils of 47-million-year-old forester moths were also examined with electron microscopes and spectrophotometry. After simulating the temperatures and pressures of fossilisation, it was concluded that these moths were a matte yellowish-green, probably corresponding with leaves in its environment – evidence of camouflage evolution to avoid detection by predators. Fossil insect colours may have served to heighten other defensive signals, or non-visual functions like thermoregulation.
Functions of Colours
Colour in modern animals can be used to predict behaviour in extinct organisms. A study in 2017 investigated the role of the tetrapyrrole molecules protoporphyrin (PP) and biliverdin (BV) in egg colouration of non-avian theropod dinosaurs. Evidence of PP and BV pigments was found in the eggshells of the Late Cretaceous oviraptor, Heyuannia huangi. This is thought to have given oviraptor eggs a blue-green colour, supporting the theory of partial open nesting oviraptorid behaviour. The study hypothesised that egg coloration evolved after the transition from burying eggs to building open and exposed nests, where eggs became visible to parents and predators, at which point selection for egg colour would have arisen. In modern birds, blue-green egg colour is intraspecifically linked to paternal care, which is thought to have occurred among oviraptorids, further supporting the blue-green egg theory.
A different study explains how electron microscopes were used to identify other colour-inducing microstructures in a 10-million-year-old snake skin from Spain. A combination of cell types in reptiles, melanophores with iridophores or xanthophores, gives green colour, which was used to conclude that the snake had green, black, and yellow-green spots, and a pale underbelly, helping to camouflage the snake among leaves to evade predators in day time like modern snakes.

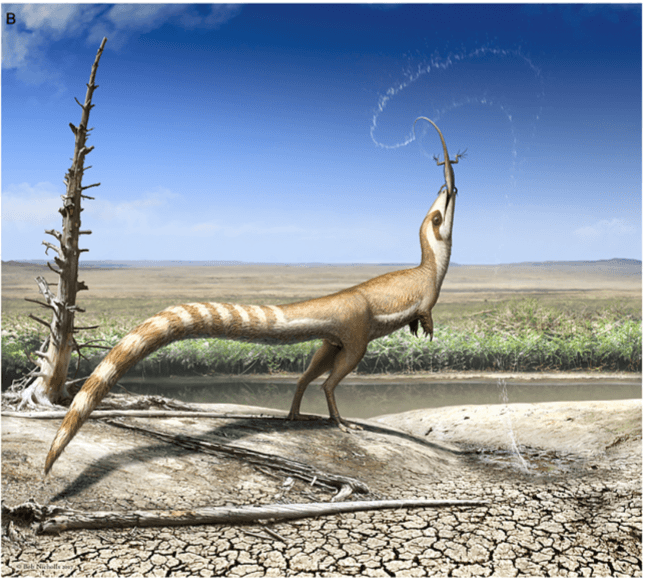
Another investigation looked at Sinosauropteryx countershading – a pattern which breaks up the body’s outline to avoid detection by predators and prey. The study explains how white feathers have no pigment so are not preserved in fossils, meaning gaps in plumage likely contained white feathers. Combined with existing knowledge from melanosomes, this information was used to map Sinosauropteryx’s body colour pattern based on its light and dark areas. Compared with modern birds, a bandit mask may have helped to hide its eyes and reduce glare, allowing it to observe potential predators and prey without being spotted. Countershading is indicative of living in an open environment due to sharp transition from dark to light colours high on its flanks, suggesting it lived in a more diverse environment than previously thought.

Scanning electron microscopes have been used to compare fossil melanosomes in Anchiornis huxleyi to those in black, grey and brown feathers of living birds, suggesting Anchiornis had black/grey feathers over most of its body, black and white feathers on its limbs, and a plume of red feathers on its head, which were likely used in social displays. The discovery of black melanin in Archaeopteryx feathers suggests they served a purpose of communication and/or thermoregulation. Similarly, dark eumelanin found in Ichthyosaurs suggests camouflage in deep oceans, like modern sperm whales, and thermoregulation at the surface, where a dark surface could have aided heat absorption while basking in sunlight, as with modern leatherback turtles.
Mammoth Colours
Researchers have been able to determine the colours of woolly mammoth fur by analysing frozen mammoth fur (such as that of 10,000-year-old juvenile Yuka), flesh, teeth, bones and tusks. The individual, Yuka, had intact ginger fur, providing evidence for the theory proposed in 2006 that mammoths could have had lighter-coloured coats rather than dark fur alone, after analysis of genes from a bone. Using polymerase chain reaction to amplify extracted DNA, researchers found that a mutant blonde allele only occurred once in 47 samples. Although pale colours may be advantageous in a snowy environment, mammoths would have spent much of the year travelling over dark, stony landscapes looking for food, with only juveniles being particularly at risk from predation. However, further research is needed to see if coat darkness varied with age or latitude. A 2014 study concluded that the popular representation of mammoths having a uniformly coloured coat may not be true to life. Closer observations of fur pigmentation revealed that mammoth coats may have been more mottled in appearance, indicating greater variation in colour.

Pinnacle of Fossilisation
It should be noted that the preservation of colour in fossils is very rare, as indicators of colouration are often destroyed/lost during fossilisation. Tissues of soft-bodied organisms can be preserved in some environments where a near absence of oxygen prevents normal decomposition. Fossil feathers, hairs and eyes tend to be preserved as carbonised traces, which form when organisms are quickly buried under low-oxygen conditions. The spherical/elongate masses found in these tissues have been established to be melanosomes, but even when preserved they may have decayed after being subjected to extreme temperatures and pressures over time, making it necessary to recreate these conditions. This can confirm how dense a fossil’s melanosomes were when the organism lived, indicating how brightly-coloured the animal’s feather (for example) was. Predicting the original shape and arrangement of melanosomes can then show if there was iridescence. Fossilised melanosomes can indicate the pigment that produced coloration in extinct organisms, along with nanostructures, tetrapyrroles and other pigment cells. The colours of extinct organisms indicate their function, such as for thermoregulation or signalling, and how they managed to fit into their environments, suggesting what these palaeoenvironments looked like.
For more information on colours found in fossils, see our articles on Sinosauropteryx and Borealopelta.
Image References
[1] Sinosauropteryx, one of the extinct animals for which colour has been determined. Artwork by Bob Nicholls.
[2] Fossilised casts of phaeomelanosomes (A) and eumelanosomes (B) from filaments on the dinosaur Sinornithosaurus. Click Here.
[3] Visual image of the bird Confuciusornis sanctus (A) and its elemental map produced by X-ray fluorescence (B). Copper is shown in red, calcium in blue and zinc in green. Black and white artistic reconstruction of the bird by Richard Hartley (C). Click Here.
[4] Table of animals in colour with illustrations by Lucy Reading. Taken from Dance (2016).
[5] Graphical Abstract taken from Smithwick, et al, 2017.
[6] An illustration of the potential variety seen in the coat of a mammoth. Image taken from Tridico, et al. (2014).
Information References and Further Sources
[1] Alger, S. J. (2015). How We Know the Colors of Prehistoric Animals. Accessed 16th July 2018. Click Here.
[2] Barden, H. E. (2012). Fossil Focus: the preservation of colour. Accessed 8th July 2018. Click Here.
[3] Benton, M. J. (2014). Vertebrate Palaeontology. 4th ed. Oxford: Wiley-Blackwell. pp. 359-360.
[4] Carney, R. M., Vinther, J., Shawkey, M. D., D’Alba, L., and Ackermann, J. (2012). ‘New evidence on the colour and nature of the isolated Archaeopteryx feather’, Nature Communications, 3 (1), pp. 1-6. Accessed 7th April 2020. Click Here.
[5] Colleary, C., Dolocan, A., Gardner, J., Singh, S., Wuttke, M., Rabenstein, R., Habersetzer, J., Schaal, S., Feseha, M., Clemens, M., Jacobs, B. F., Currano, E. D., Jacobs, L. L., Sylvestersen, R. L., Gabbott, S. E., and Vinther, J. (2015). ‘Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils’, Proceedings of the National Academy of Sciences of the United States of America, 112 (41), pp. 12592–12597. Accessed 7th April 2020. Click Here.
[6] Dance, A. (2016) ‘News Feature: Prehistoric animals, in living color’, PNAS, 113 (31), pp. 8552-8556. Accessed 7th April 2020. Click Here.
[7] Digital Atlas of Ancient Life. (Unknown). Types of fossil preservation. Accessed 16th July 2018. Click Here.
[8] Furness, H. (2012). Discovered: woolly mammoth with ‘strawberry-blonde hair’, The Telegraph. Accessed 8th July 2018. Click Here.
[9] Glass, K., Ito, S., Wilby, P. R., Sota, T., Nakamura, A., Bowers, C. R., Miller, K. E., Dutta, S., Summons, R. E., Briggs, D. E. G., Wakamatsu, K., and Simon, J. D. (2013). ‘Impact of diagenesis and maturation on the survival of eumelanin in the fossil record’, Organic Geochemistry, 64, pp. 29-37. Accessed 7th April 2020. Click Here.
[10] Lindgren, J., Uvdal, P., Sjövall, P., Nilsson, D.E., Engdahl, A., Schultz, B.P., and Thiel, V. (2012). ‘Molecular preservation of the pigment melanin in fossil melanosomes’, Nature Communications, 3 (1), pp. 1-7. Accessed 7th April 2020. Click Here.
[11] McNamara, M. E., Briggs, D. E. G., Orr, P. J., Noh, H., and Cao, H. (2012). ‘The original colours of fossil beetles’, Proceedings of the Royal Society B: Biological Sciences, 279 (1731), pp. 1114–1121. Accessed 7th April 2020. Click Here.
[12] McNamara, M. E. (2013). ‘The Taphonomy of Colour in Fossil Insects and Feathers’, Palaeontology, 56 (3), pp. 557-575. Accessed 7th April 2020. Click Here.
[13] Russell, P., Hertz, P., and McMillan, B. (2011). Biology: The Dynamic Science, Volume 2. Belmont, California (US): Brooks/Cole. Cengage Learning. pp. 474.
[14] Smithwick, F. (2017). ‘We discovered this dinosaur had stripes – and that tells us a lot about how it lived’, The Conversation. Accessed 8th July 2018. Click Here.
[15] Smithwick, F. M., Nicholls, R., Cuthill, I. C., Vinther, J. (2017). ‘Countershading and Stripes in the Theropod Dinosaur Sinosauropteryx Reveal Heterogeneous Habitats in the Early Cretaceous Jehol Biota’, Current Biology, 27 (21), pp. 3337-3343.e2. Accessed 7th April 2020. Click Here.
[16] Sloan, C. (2010). ‘Dinosaur True Colors Revealed for First Time’, National Geographic. Accessed 8th July 2018. Click Here.
[17] Vinther, J. (2016). ‘Fossil melanosomes or bacteria? A wealth of findings favours melanosomes’, Bioessays, 38 (3), pp. 220-225. Accessed 7th April 2020. Click Here.
[18] Tridico, S. R., Rigby, .P, Kirkbride, K. P., Haile, J., and Bunce, M. (2014). ‘Megafaunal split ends: microscopical characterisation of hair structure and function in extinct woolly mammoth and woolly rhino’, Quaternary Science Reviews, 83, pp. 68-75. Click Here.
[19] Virginia Tech. (2015). ‘Pigment from fossils identified, revealing color of extinct animals’, Science Daily. Accessed 8th July 2018. Click Here.
[20] Wiemann, J., Yang, T., Sander, P.N., Schneider, M., Engeser, M., Kath-Schorr, S., Müller, C.E., and Sander, P.M. (2017). ‘Dinosaur origin of egg color: oviraptors laid blue-green eggs’, PeerJ, 5. Accessed 7th April 2020. Click Here.